
- #CHARGE TRANSFER CALCULATION USING MULIKEN QUANTUMWISE HOW TO#
- #CHARGE TRANSFER CALCULATION USING MULIKEN QUANTUMWISE SOFTWARE#

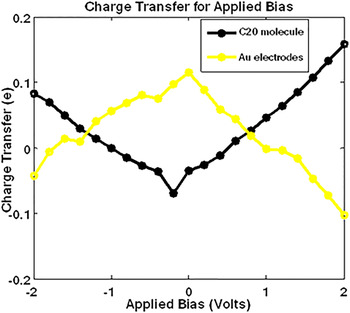
The above text leaves me to believe the method of determining the degree of charge transfer is as follows: Which may be defined as the sum of all atomic charges on the Of charge transfer between the donor and acceptor molecules, The calculated atomic charges were then used to obtain the degree who gives more details on a similar method, writing on page 2, left column:
#CHARGE TRANSFER CALCULATION USING MULIKEN QUANTUMWISE HOW TO#
I do not know how to obtain the degree of charge transfer from the resulting population analysis. (bottom of page 9 in the Theory footnote). In figure 3b, the authors show the correlation between the degree of charge transfer, CT $_$ refers to a population analysis such as a Mulliken or natural population (AKA Lowdin population), referenced in Mendez et al. I am interested in section 3.2 in the main text about using the bond length as an estimate for the charge transfer amount. Not aware of other programs implementing similar schemes, so you have to find one on your own.I am trying to reproduce the quantity referred to as the degree of charge transfer reported in this publication by Zhu et al. However, if it is not an option, analog to Gaussian Pop=CHelpG should be used, that fit atomic charges to produce electrostatic potential closest to one produced for given electronic distribution. Ideally you should use full-scale electrostatic potential. Given you care to use the charges to model electrostatic potential, Mulliken and Natural charges go to garbage. It is generally recomended to employ periodic approach using plane-wave sets, possibly in DFT method, as it greately reduces memory requirements and amount of atoms (and electrons) considered. However, ideally you should perform several computations, gradually increasing amount of layers in slab until the characteristic you considering stabilize. Generally, two-three layers of atoms close to surface are 'relaxed' and two-three 'deeper' ones are fixed in positions, modelling unperturbed crystal structure.
Generally, oxides tend to adsorb water forming hydroxide groups and some other small molecules.Īfter that you have to build a model slab of the surface. Second, you have to consult literature to find which surface species exist on the surface in your conditions. $\ce$ may exhibit several different faces, you'll have to consult literature to find out which are the most frequent. I think the latter (electrostatic potential fitting) will be better generally for molecular dynamics, since you're attempting to produce point charges that will best represent the quantum mechanical electrostatic potential.įirst, you have to decide, what face you are working with. My personal preference is for something like Hirshfeld charges (i.e., from the electron density, so not basis-set dependent), or electrostatic potential fitting schemes like Merz-Kollman or CHelpG.
#CHARGE TRANSFER CALCULATION USING MULIKEN QUANTUMWISE SOFTWARE#
There are lots, and I mean lots of methods and software programs to produce partial charges.


 0 kommentar(er)
0 kommentar(er)
